Hydrogen has an ionization potential of 13.6 ev. Determine the absolute energy difference in eV between the n = 1 and the n = 6 energy levels of a hydrogen atom. Do not include units please. (at least not in the final answer). Question: 2-2 Where Hyu=-13.6 EV. And Hyp=-1.758 Huu Hyp Part A Evaluate The Energies Betting, Santibending For Sup = 0.070 Express Your Answer In Electron-volts To Three Significant Figures Separated By A Comma. The energy needed to free the electron 1) The energy of hydrogen atom is E = - in the ground state is: a) 1.51eV b) 3.4 eV c) 13.6 eV d) 0 2) The transition 43P to 43D in Orthohelium is: a) Allowed. B) Forbidden c) Sometimes allowed. D) Allowed if electrons stop spinning, 3) The transition 435 to 4D in Orthohelium is: a) Allowed. The kinetic and potential energies are 13.6 eV and -27.2 eV respectively. Similar Questions. Write laws of refraction Explain the same with the help of ray diagram when a ray of light passes through a rectangular glass slab Q. Two stones are thrown vertically upwards simultaneously with their initial velocities Q.
The energy of an electron in 1st bohr orbit of H atom is -13.6 eV. The possible energy value of electron in excited state of doubly ionised Lithium is what?
1 Answer
13.6 Ev In Joules
Explanation:
Energy of an electron in hydrogen like species in
For electron in first orbit of
For electron in second orbit (
Energy of electron in an orbit after
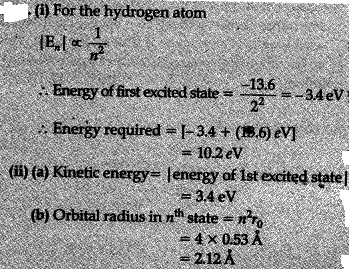
Related questions
In physics and chemistry, the Lyman series is a hydrogen spectral series of transitions and resulting ultravioletemission lines of the hydrogenatom as an electron goes from n ≥ 2 to n = 1 (where n is the principal quantum number), the lowest energy level of the electron. The transitions are named sequentially by Greek letters: from n = 2 to n = 1 is called Lyman-alpha, 3 to 1 is Lyman-beta, 4 to 1 is Lyman-gamma, and so on. The series is named after its discoverer, Theodore Lyman. The greater the difference in the principal quantum numbers, the higher the energy of the electromagnetic emission.
History[edit]
The first line in the spectrum of the Lyman series was discovered in 1906 by Harvard physicist Theodore Lyman, who was studying the ultraviolet spectrum of electrically excited hydrogen gas. The rest of the lines of the spectrum (all in the ultraviolet) were discovered by Lyman from 1906-1914.The spectrum of radiation emitted by hydrogen is non-continuous or discrete. Here is an illustration of the first series of hydrogen emission lines:
Historically, explaining the nature of the hydrogen spectrum was a considerable problem in physics. Nobody could predict the wavelengths of the hydrogen lines until 1885 when the Balmer formula gave an empirical formula for the visible hydrogen spectrum. Within five years Johannes Rydberg came up with an empirical formula that solved the problem, presented first in 1888 and in final form in 1890. Rydberg managed to find a formula to match the known Balmer series emission lines, and also predicted those not yet discovered. Different versions of the Rydberg formula with different simple numbers were found to generate different series of lines.
On December 1, 2011, it was announced that Voyager 1 detected the first Lyman-alpha radiation originating from the Milky Way galaxy. Lyman-alpha radiation had previously been detected from other galaxies, but due to interference from the Sun, the radiation from the Milky Way was not detectable.[1]
The Lyman series[edit]
The version of the Rydberg formula that generated the Lyman series was:[2]
13.6 Ev Hydrogen
Where n is a natural number greater than or equal to 2 (i.e., n = 2, 3, 4, …).
Therefore, the lines seen in the image above are the wavelengths corresponding to n = 2 on the right, to n = ∞ on the left. There are infinitely many spectral lines, but they become very dense as they approach n = ∞ (the Lyman limit), so only some of the first lines and the last one appear.
The wavelengths in the Lyman series are all ultraviolet:
| n | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ∞, the Lyman limit |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wavelength (nm) | 121.56701[3] | 102.57220[3] | 97.253650[3] | 94.974287[3] | 93.780331[3] | 93.0748142[3] | 92.6225605[3] | 92.3150275[3] | 92.0963006[3] | 91.9351334[3] | 91.1753 |
Explanation and derivation[edit]
In 1914, when Niels Bohr produced his Bohr model theory, the reason why hydrogen spectral lines fit Rydberg's formula was explained. Bohr found that the electron bound to the hydrogen atom must have quantized energy levels described by the following formula,
According to Bohr's third assumption, whenever an electron falls from an initial energy level Ei to a final energy level Ef, the atom must emit radiation with a wavelength of
There is also a more comfortable notation when dealing with energy in units of electronvolts and wavelengths in units of angstroms,
- Å.
Energy Cf 70 Speaker Review
Replacing the energy in the above formula with the expression for the energy in the hydrogen atom where the initial energy corresponds to energy level n and the final energy corresponds to energy level m,
Where RH is the same Rydberg constant for hydrogen from Rydberg's long known formula. This also means that the inverse of the Rydberg constant is equal to the Lyman limit.


For the connection between Bohr, Rydberg, and Lyman, one must replace m with 1 to obtain
which is Rydberg's formula for the Lyman series. Therefore, each wavelength of the emission lines corresponds to an electron dropping from a certain energy level (greater than 1) to the first energy level.
See also[edit]
References[edit]
- ^'Voyager Probes Detect 'invisible' Milky Way Glow'. National Geographic. December 1, 2011. Retrieved 2013-03-04.CS1 maint: discouraged parameter (link)
- ^Brehm, John; Mullin, William (1989). Introduction to the Structure of Matter. John Wiley & Sons. p. 156. ISBN0-471-60531-X.
- ^ abcdefghijKramida, A., Ralchenko, Yu., Reader, J., and NIST ASD Team (2019). NIST Atomic Spectra Database (ver. 5.7.1), [Online]. Available: https://physics.nist.gov/asd [2020, April 11]. National Institute of Standards and Technology, Gaithersburg, MD. DOI: https://doi.org/10.18434/T4W30F




